Blog
Pinnacle 21 Releases P21 Community 4.1.0
Release Date: May 31, 2024
P21 Community 4.1.0 is now available! Download the latest version.
What's New in P21 Community 4.1.0?
This release brings significant updates to support the transition to the Dataset-JSON format for clinical trial submissions.
Proposed by CDISC and PHUSE, Dataset-JSON addresses many of the limitations of XPT format, such as character encoding, variable naming/sizing, and storage efficiency. Dataset-JSON also expands the options for extensible data tables and integrated metadata, improving the consistency and reusability of clinical research data.
In collaboration with CDISC and PHUSE, the FDA is conducting a pilot project to ensure that the industry can transition to Dataset-JSON. Starting with workshops, hackathons, and proof-of-concept tests, the goal is to establish Dataset-JSON as a new standard for regulatory submissions, ultimately replacing XPT.
JSON is already a widely accepted data standard that works with almost any programming language. Since it removes some of the limitations of XPT format, it adds greater capacity for future development of clinical data standards.
Dataset-JSON was adapted from the Dataset-XML Version 1.0 specification. Read more about the new Dataset-JSON specification from CDISC.
To support the transition to Dataset-JSON, we’ve made the following improvements to P21 Community:
- Validator: The Validator now supports source files that are in Dataset JSON format.
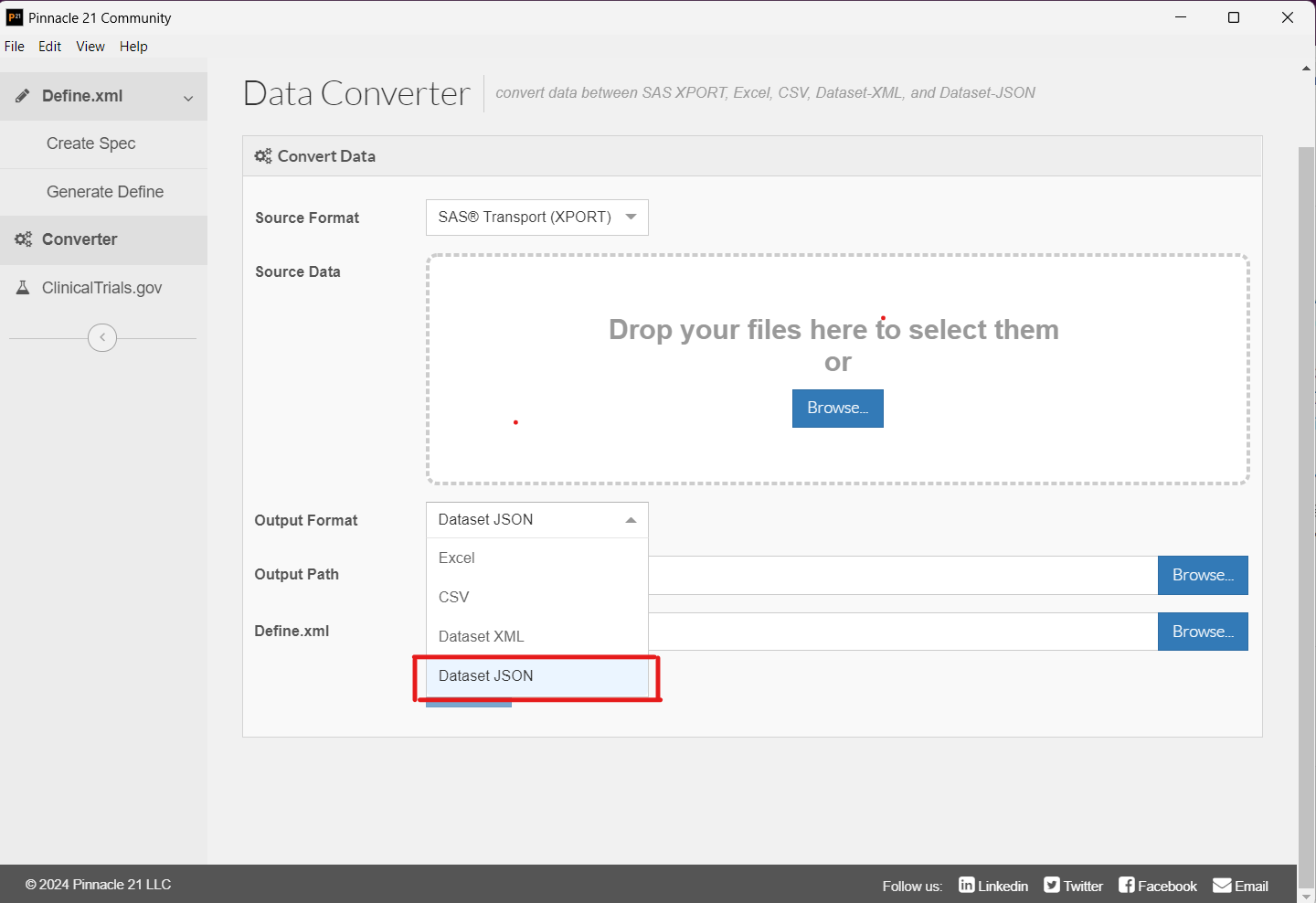
- Converter: The Data Converter now includes the option to convert your SAS XPT files to Dataset-JSON format.
- Define.xml Generator: This release includes several bug fixes to support Dataset-JSON:
- Generating a spec from Define.xml now creates an Excel file which contains Standards without hyphens in the name
- Generating a Define.xml from an Excel spec now references Standards with a hyphen for Define-XML 2.0, and without a hyphen for Define-XML 2.1
- Generating a spec or Define.xml that includes a dataset with a number in the 2nd position in the name (e.g., X6) no longer produces an error
What else is new in P21C 4.1.0?
Be on the lookout for our upcoming webinar detailing all of the improvements to P21 Community in version 4.1.0, including specifics about Dataset-JSON, engine updates, and more.
Tags:
Blog Main Page




