DataFit
In August 2020, we released our new Pinnacle 21 validation engine “FDA (1907.2)” for preparing study data for FDA submissions. It includes the validation rules currently used by FDA’s DataFit, the agency’s implementation of Pinnacle 21 Enterprise.
The previous, outdated version of the validation rules is represented by our “FDA Legacy (1903.1)” engine. It will be available for the next few months until our next release. This window allows you to finalize your ongoing submission preparations.

In late 2014, the FDA announced that, starting December 17, 2016, all new clinical and nonclinical studies must be submitted electronically and contain data in conformance with the standards specified in FDA’s Data Catalog. This is part of an effort to accelerate the regulatory review process.
At the time of this writing, that deadline is only nine months away. So, the big question looms: Are you 100% ready for FDA submission? Because when December 17th comes, any doubt you have may represent a risk of slowing down the review process. More importantly, you’ll be missing out on an opportunity to get your new drug to market faster than ever before.
Pinnacle 21 is pleased to announce that we’ve been awarded a renewed contract to provide software and services in ongoing support of the FDA’s DataFit program.
According to the FDA’s Center for Drug Evaluation and Research (CDER), which made the decision:

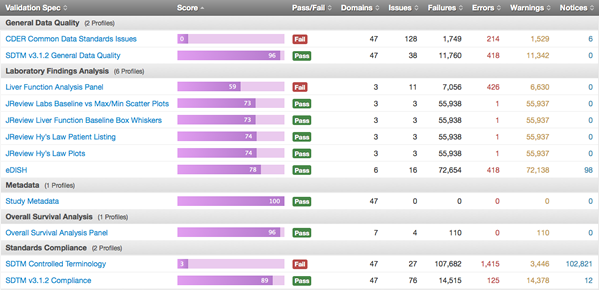
There has been a lot of buzz about DataFit of late. The FDA announced it as a new project that will enable the organization to “effectively leverage standard data to advance the review process.” But this new project is more than ten years in the making.





