Regulatory reviewers want a handy compass to orient themselves around your data. In practice, this "want" is really a "need." Things happen. Sometimes you extend controlled terminology. Sometimes a subject gets enrolled twice in the same study. Or maybe some validation rules don't yet apply since the study is still ongoing...
It's best to note these needed yet non-conformant data upfront. Advocate for your data and share their full story in our xDRG creator. Our tool automates your content aggregation and formatting. Push one button, and, behold, a thorough, persuasive Reviewer’s Guide appears!
Pre-populate your Reviewer’s Guide template with trial design and summary, subject data, study metadata, and validation results. We do the formatting for you.
Simplify your process. Works with clinical, non-clinical, and analysis data (SDTM, SEND, and ADaM).
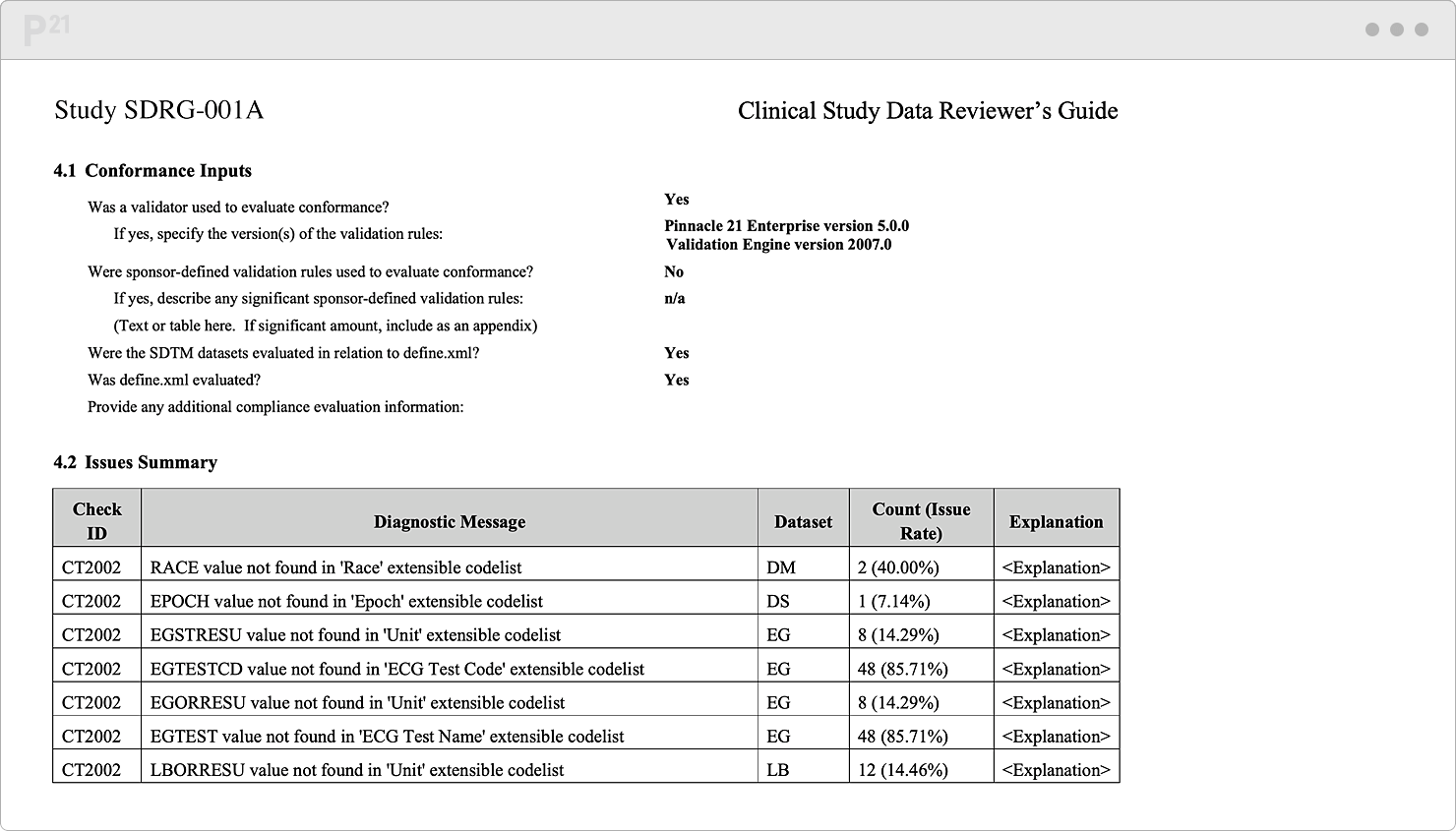

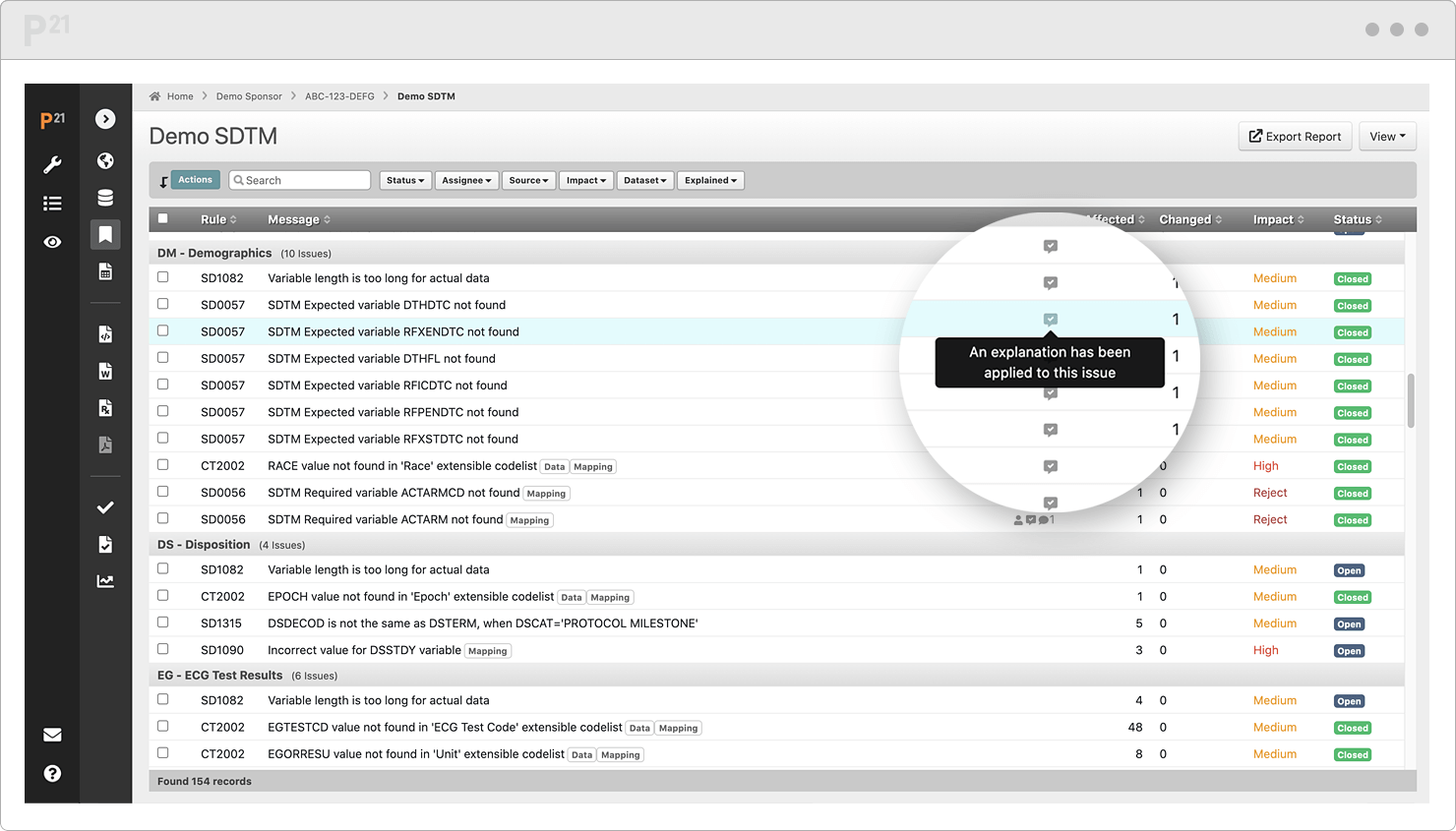
Stop chasing down issue explanations two weeks before submission. Upcycle your existing content from throughout the study lifecycle.
Tell your data’s story well, once, and only once. Your team already canonized the explanations in our Issue Management module, so it's a "twofer."