Blog
FDA DataFit: an introduction
There has been a lot of buzz about DataFit of late. The FDA announced it as a new project that will enable the organization to “effectively leverage standard data to advance the review process.” But this new project is more than ten years in the making.
Back in 2002, when FDA launched its 21st Century Review Initiative, it sought to establish aset of performance standards to be followed during drug review. One of FDA’s goals was to make the submission process more efficient and standardized. It wanted reviewers to spend less time analyzing the quality of data (or lack thereof) upon submission, so they could spend more time exploring and considering the contents of the study data itself.
High quality standardized data became the key to the FDA meeting its goals.
The FDA defines “Data Quality” as that which is both compliant and useful. The need for standardized, compliant data was the impetus for the establishment of CDISC standards. But the usefulness of data was an issue that still needed to be addressed. What good was a set of standards if the data being submitted didn’t support its intended use?
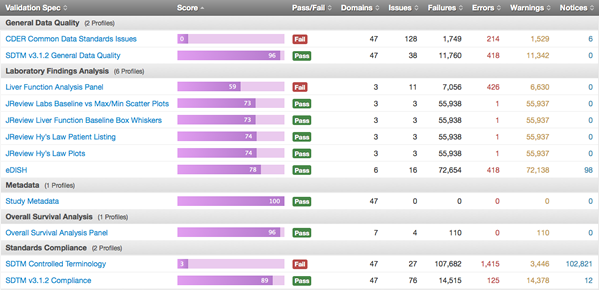
To address the problem comprehensively, FDA launched the DataFit project. The project’s goal, quite simply, is to enable FDA reviewers to rapidly assess whether submitted standard data is “fit for use.” Through DataFit — specifically, through a combination of software and implementation — a detailed assessment of submitted data is performed very early in the review process, based on data requirements for identified review activities.
The software that drives the FDA DataFit project is OpenCDISC Enterprise. This software validates all new submissions that come in. A team at FDA, which includes professionals from Pinnacle 21, reviews and analyses these reports. These help reviewers understand, immediately, if there are any data-quality issues that could prevent them from doing their job.
More than a decade after FDA threw down the gauntlet with the 21st Century Review Initiative, its vision has come full circle. Through the DataFit project, reviewers can envision a future where all new submissions will be delivered “fit for use” — so they can dedicate their time to the review process itself. And sponsoring pharmaceutical companies will have a way to side-step the various issues associated with poor data quality, and help get their new products approved and to-market more quickly.
Tags:
Blog Main Page




